131
Views & Citations10
Likes & Shares
MATERIALS AND METHODS
Materials
Chloroquine phosphate, PEG 4000,400 is collected from FDC Limited, Mumbai. Coconut oil, white bees wax, Methyl paraben, Propyl paraben Cocoa butter, Vanaspati were collected from Fine chemical Industry, Mumbai.
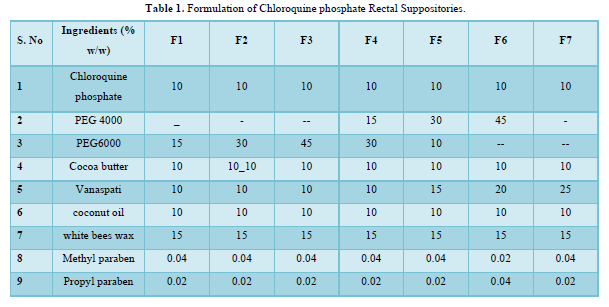
Preparation of Chloroquine phosphate Suppositories
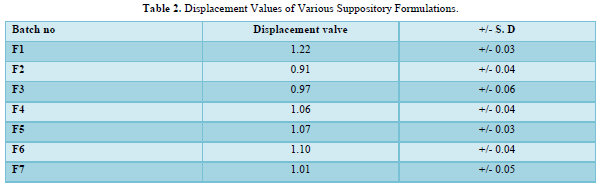
The displacement value of CQP with different bases was determined. A calculated quantity of CQP, finely shifted was added to the molten base (water bath heated) and stirred continuously until just pourable and poured into 1g capacity suppository molds to overflow. Finally, Vanaspati was incorporated at 75-80℃ and mixed thoroughly. The molten mass was poured into earlier calibrated by stainless steel mold of 1gm and allowable to set and kept in freeze for cooled for a little time and removed it. The PEG suppositories were prepared by using fusion method by melting PEG (4000, and 6000) in various concentrations and then drug was incorporated. Cocoa butter suppositories were prepared by melting bees wax and cocoa butter on water bath and then the drug was incorporated [8]. The details of all formulations are tabulated in Table 1 & 2. The formulated suppositories were packed in Aluminum foil kept in refrigerator and used in the evaluation [9].
Calibration of Mold
Calibrating the molds is important because different molds may have varying capacities, which can affect the weight and consistency of the suppositories, produced. The process you described involves melting the base material, filling it into the mold, and then weighing the mold after removing the suppositories. The average weight obtained from multiple repetitions of this process is considered the true capacity of the mold. According to the information you provided, the calibrated mold capacities ranged from 1.02 to 1.195 grams. This suggests that different molds were tested, and their capacities fell within this weight range. It's important to note that the specific range of calibrated mold capacities may vary depending on the type of suppository base and the manufacturer of the molds. Calibrating the molds ensures consistent and accurate dosing when producing suppositories, which is essential for effective and safe medication administration [10].
Evaluation of Suppositories
Weight Variation
If any suppository deviates from the average weight by more than 5%, it is considered to be out of specification. To illustrate this, let's assume that the average weight of the suppositories is 10 grams. According to the requirements, any suppository should not deviate from this average weight by more than 5%. If we consider a 5% deviation, the acceptable weight range for each suppository would be between 9.5 grams (10 grams - 0.5 grams) and 10.5 grams (10 grams + 0.5 grams). However, there are two exceptions allowed, which means that these two suppositories can deviate from the average weight by up to 5%. In this case, the weight range for these two suppositories would be between 9.5 grams (10 grams - 0.5 grams) and 10.5 grams (10 grams + 0.5 grams), just like the others. If any suppository weighs less than 9.5 grams or more than 10.5 grams (excluding the two exceptions), it would be considered out of specification [11].
Hardness (Fracture Point)
The hardness of prepared suppositories can be tested using a Monsanto hardness tester model. In this test, the weight required for a suppository to collapse is measured, which serves as an indicator of its hardness. This measurement helps assess the suppository's ability to withstand packing and transportation hazards. Additionally, a hardness test, also known as a fracture point test, can be performed to determine the tensile strength of the suppositories. During the hardness test, a suppository is subjected to an increasing force until it breaks or fractures. The point at which the suppository fractures indicates its tensile strength, which is a measure of its ability to resist deformation or breakage. This test provides valuable information about the suppository's structural integrity and its capacity to withstand external stress during handling, packaging, and transportation processes. By evaluating the hardness and tensile strength of suppositories, manufacturers can ensure that the products remain intact and retain their desired shape and consistency throughout their lifecycle, minimizing the risk of damage or alteration during transit [12].
Disintegration Test
The disintegration test was conducted on suppositories using the USP (United States Pharmacopeia) tablet disintegration test apparatus called Electro lab, ED 2L. The test was performed on six suppositories of each type. For suppositories prepared with water-soluble bases, the time required for complete disintegration was determined. To conduct the test, 160ml of distilled water was used as the medium, and the temperature was maintained at 37°C (body temperature). The suppositories were placed in the disintegration test apparatus, and the time it took for them to completely disintegrate in the water was recorded for each sample. For suppositories prepared with oily bases, the time required for complete disintegration was also determined [13]. The same procedure was followed as for water-soluble suppositories, with 160ml of distilled water at 37°C used as the medium. However, in the case of oily suppositories, the time it took for them to fully disintegrate in the water was measured. The purpose of this test is to assess the ability of the suppositories to disintegrate in the intended environment, ensuring that they can release the active ingredients effectively once inserted rectally or vaginally. The specific disintegration times for each type of suppository will help evaluate their formulation and determine their suitability for use [6].
Macro Melting Range Test
For the macro melting range test, the sample is filled to a height of 1cm in a 10cm long capillary tube and placed in a beaker of water. Increase the temperature gradually and record the temperature of the liquefied product [14].
Liquefaction time and temperature
It is done using the apparatus manufactured for liquefaction time and temperature. Use a large straw with a narrow mouth on one side and a wide mouth on the other. Immerse the straw in the controlled water temperature of 37°C. So, the narrow end faces the hot water. Route the sample wick from the top of the straw from the wide end and carefully push until it reaches the narrow end. A glass rod is placed on top of the wick. Record the temperature of the newly dropped glass rod, which represents the liquefaction temperature. After the wick is completely melted, the time it takes for the glass rod to reach the narrow end is the liquefaction time [15].
Drug Content
The suppositories are taken and dissolved in 100 ml of phosphate buffer with a pH of 7.4. This is done by stirring the mixture slowly using a magnetic stirrer. Temperature and Time: The stirring process is carried out at a temperature of 37°C (body temperature) for duration of 1 h. This allows the suppositories to dissolve completely in the buffer solution. Filtration: Once the stirring is complete, the solution is filtered to remove any insoluble particles or impurities present in the mixture. This step ensures that only the dissolved drug is analyzed further. Dilution: The filtrate, which contains the drug from the dissolved suppositories, is suitably diluted. The purpose of dilution is to ensure that the concentration of the drug falls within the linear range of the instrument used for measurement [16]. Absorbance Measurement: The diluted solution is then subjected to absorbance measurement at a wavelength of 371 nm. Absorbance is a measure of how much light is absorbed by a substance at a specific wavelength. Blank Measurement: A blank sample is prepared using the same procedure but without the suppositories. This blank solution serves as a reference to account for any background absorbance caused by the buffer solution itself. Data Analysis: The absorbance of the diluted solution containing the drug is measured against the absorbance of the blank solution. The difference between these absorbance values provides information about the drug content in the suppositories. The specific details and parameters mentioned in the passage, such as pH 7.4 and 371 nm, may vary depending on the drug being analyzed and the analytical method employed [17].
Dissolution Test
Dissolution Apparatus: The USP tablet dissolution test apparatus used for the study is the Electro lab -TDT 08L. Specifically, a rotating basket apparatus of Type I was employed. Rotational Speed: The basket was rotated at a speed of 100 rpm (revolutions per minute). Dissolution Medium: Phosphate buffer with a pH of 7.4 was used as the dissolution medium Volume of Dissolution Medium: A total of 500 ml of the phosphate buffer was used. Temperature: The dissolution medium was maintained at a temperature of 37±0.5°C. Time Intervals: At predetermined time intervals, 5 ml samples were withdrawn from the dissolution medium. Collection Method: A syringe fitted with a pre-filter was used to withdraw the samples. Filtration: The withdrawn samples were filtered through Whatman filter paper. Replacement of Volume: After withdrawing each sample, the same quantity of fresh dissolution medium was added to maintain a constant volume in the dissolution vessel. Temperature Maintenance: The replacement medium was also maintained at 37±0.5°C. Analysis: Measurement Method: The samples were analyzed for drug release by measuring the absorbance at 371 nm. Instrumentation: A UV-visible spectrophotometer was used for absorbance measurements each test used one suppository [18]. The absorbance measurements were taken at different predetermined time intervals. Replicates: The dissolution studies were performed in triplicate, meaning the entire process was repeated three times (n=3). The obtained data, representing the cumulative percent of Chloroquine phosphate released over time, was calculated and plotted to visualize the release profile [19]. Please note that this summary is based on the information you provided, and additional details or clarifications may be necessary for a more comprehensive understanding of the study [20].
Stability Studies
A short-term safety study was performed at room temperature and on contracted formulations stored in cold storage (4°C) for 6 weeks. Suppositories are wrapped in foil and packed in a cardboard box. Samples were taken after 6 weeks for dissociation tests to estimate drug content and determine drug release profile.
RESULTS
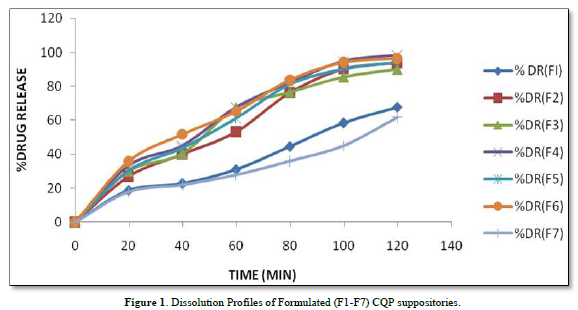
The provided information describes the results of various tests and studies conducted on different types of suppositories containing Chloroquine phosphate. Here are the key findings: Weight Variation: The weight of all the prepared suppositories fell surrounded by the up to standard range of <5%, indicating that the mold calibration was ideal. Uniformity of Drug Content: The drug content in all the suppositories showed uniformity within the permissible range of 95 to 100%, suggesting that the drug was evenly dispersed in the suppositories. Mechanical Strength: The suppositories demonstrated good mechanical strength with an optimum hardness ranging from 1.2 to 2.1 kg/cm2.In PEG suppositories, raising the concentration of PEG 4000, 6000 better mechanical strength. In cocoa butter suppositories, mechanical strength increased with higher amounts of beeswax up to 3% w/w, further than which brittleness occurred. Macro Melting Range: The macro melting range test was performed to determine the temperature range at which the suppositories melted. Chloroquine phosphate suppositories had a macro melting range of 51 to 63℃. In PEG suppositories, the macro melting range better with higher amounts of PEG 6000 and PEG 4000 (54 to 56℃). In cocoa butter suppositories, the macro melting range was 40 to 44℃ and increased with increased beeswax concentration. Liquefaction Time: Liquefaction time refers to the time required for the suppository to liquefy under rectal pressure. For Vanaspati-based suppositories, liquefaction temperature ranged from 52 to 58℃, and liquefaction time ranged from 8 to 12 min. In PEG suppositories, liquefaction temperature ranged from 46 to 50℃, and liquefaction time ranged from 12 to 20 min with increasing amounts of PEG 4000 and PEG 6000. In cocoa butter suppositories, liquefaction temperature ranged from 36 to 40℃, and liquefaction time ranged from 4 to 6 min with increased beeswax amount. Disintegration Test: Chloroquine phosphate-based suppositories took more than 9 min to disintegrate. PEG suppositories disintegrated within a time period of 6 to 9 min, indicating that PEG was an effective disintegrant. Cocoa butter suppositories lost their shape within 6 to 7 min. In vitro Release Profile: Tables 3 & 4 provides information on the release of Chloroquine phosphate from different bases. The combination of PEG and Vanaspati suppositories showed more than 50% drug release in dissolution studies, indicating drug diffusion from the hydrophilic matrix over time. Absorption Enhancement: Addition of coconut oil (10% w/w) and beeswax (15% w/w) to Chloroquine phosphate-based suppositories enhanced drug absorption significantly the drug release study profile was shown in the Figure 1. This effect may be attributed to the addition of absorption enhancers. Altering the composition by adding absorption enhancers and surfactants could potentially achieve a suppository formulation with optimal drug release and improved bioavailability. Please note that the information provided is a summary of the findings presented and does not include the complete details of the study.
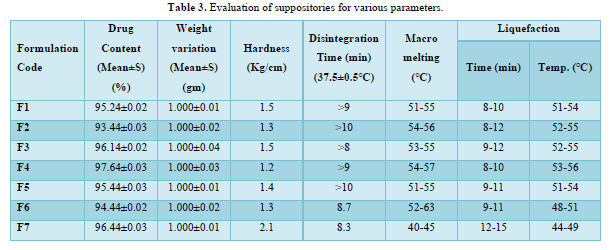
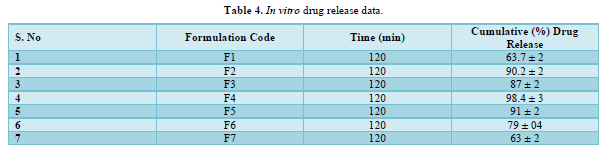
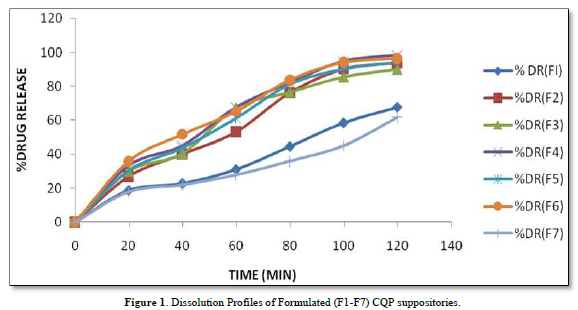
PEG Suppositories: These suppositories showed a maximum drug release of 98.3% within 120 min. The drug is released as the PEG base progressively dissolves in the dissolution media. Cocoa Butter Suppositories: The drug release from cocoa butter suppositories was slow, taking approximately 2 h. This slow release may be attributed to the high lipophilicity of the base, its non-miscibility with the dissolution media, and the absence of additives or surface-active agents. Beeswax: The addition of beeswax to cocoa butter suppositories increased the drug release. This enhancement may be attributed to the increase in hardness and the alteration of liquefaction temperature and time. Mechanism of Drug Release: The drug release from the suppositories was primarily diffusion-controlled, following first-order kinetics. The release from Vanaspati suppositories followed the Peppas kinetic model, while release from cocoa butter and PEG suppositories followed the Higuchi, Peppas, and first-order kinetic models. Stability Studies: The stability studies conducted on the Chloroquine phosphate suppositories indicated that there were no significant changes in the drug content, physical characteristics, and dissolution profile after storing them for 6 weeks at both refrigeration and room temperature. The suppositories maintained satisfactory hardness, macro-melting test, liquefaction test, and disintegration properties for practical use.
In summary, the PEG suppositories exhibited a fast drug release, while the cocoa butter suppositories showed slow release, which was improved by the addition of beeswax. The drug release mechanisms followed diffusion-controlled kinetics, and the stability studies demonstrated the suitability of the Chloroquine phosphate suppositories for practical use.
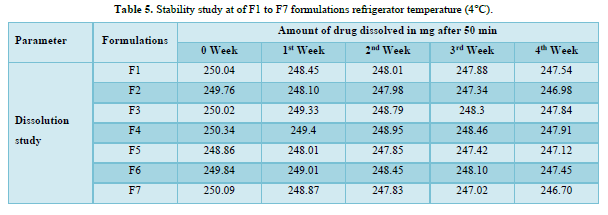
Stability studies
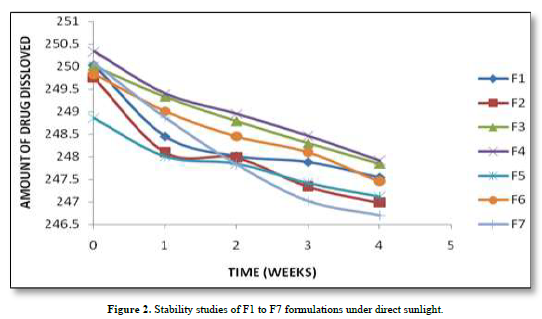
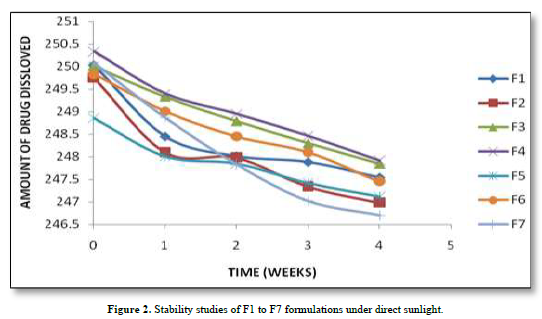
Based on the stability study results at different storage conditions, the following observations can be made: All the formulations (F1 to F7). Table 5 showed no significant difference in percentage drug content, disintegration time, and dissolution profiles at the end of 4 weeks when stored under room temperature (28°C), refrigerator temperature (4°C), and direct sunlight. Among all the formulations, F4 exhibited the least change in drug content after 4 weeks, indicating higher stability compared to other formulations. Formulation F7 showed a significant reduction in drug content after 4 weeks of storage under all three conditions (room temperature, refrigerator temperature, and direct sunlight). This suggests that F7 is less stable compared to other formulations. Disintegration time and dissolution profiles were not significantly affected by the storage conditions for any of the formulations. Therefore, based on the stability study results, formulation F4 can be considered the most stable, as it demonstrated minimal changes in drug content after 4 weeks of storage under various conditions. The stability study profile was shown in Figure 2.
In vivo Studies for the Formulated Suppositories
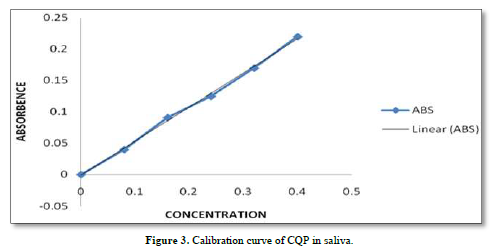
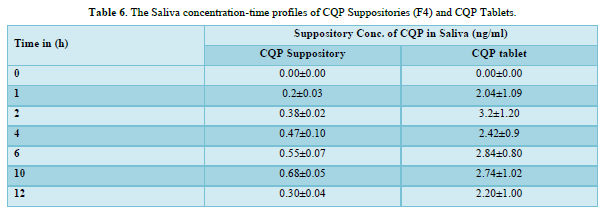
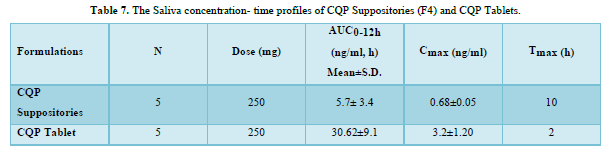
CQP tablets, and CQP suppositories, were well tolerated by the subjects, and no adverse effects were observed. Additionally, there were no instances of suppository expulsion, rectal irritation, or diarrhea reported. The study compared the mean saliva concentration versus time profiles of CQP in volunteers after a single oral dose of CQP tablets and a single rectal dose of CQP suppositories. The results showed that the peak saliva concentration (Cmax) for CQP suppositories was about 5 times lower compared to CQP tablets. CQP suppository achieved its Cmax after ten hours of rectal administration, with a value of 0.68±0.05 ng/ml. In contrast, CQP tablets reached their Cmax after 2 h of oral administration, with a higher value of 3.2±1.20 ng/ml. The area under the curve (AUC) from 0 to 12 h, a measure of drug exposure, was significantly lower for CQP suppositories (5.7±3.4 ng/ml.h) compared to CQP tablets (30.62±9.1 ng/ml.h). This indicates that the bioavailability of the formulated CQP suppository (F4), made with a PEG (4000+6000) base, is approximately 5 times lower than that of the existing CQP tablet formulation. To improve the bioavailability of the suppository formulation, the study suggests considering the addition of absorption enhancers and surfactants. These additives may help enhance the absorption of the drug from the suppository, thereby increasing its bioavailability and efficacy. The results or shown in Tables 6 & 7 absorbance curve shown in Figure 3.
CONCLUSION
Based on the information provided, it can be concluded that in the study conducted, Chloroquine phosphate suppositories were prepared using a combination of Cocoa Butter and Vanaspati bases, similar to the PEG and base used in conventional rapid release formulations. Among the seven formulations tested, F-4 demonstrated better performance in terms of disintegration time, liquification time, and dissolution rate. However, despite F-4 exhibiting the highest dissolution rate, the concentration of Chloroquine in saliva was found to be lower than the therapeutic concentration and significantly lower than that achieved with tablets. Based on this observation, it can be inferred that the suppository formulation used in the study may not be as therapeutically effective as the tablet formulation. To explore the rectal route for Chloroquine therapy, the addition of surface-active agents and absorption enhancers was found to be essential. These additives could potentially improve the absorption and bioavailability of Chloroquine when administered rectally. It's important to note that this conclusion is based on the specific study described in your statement. The effectiveness of Chloroquine phosphate suppositories compared to tablets may vary depending on various factors such as formulation, dosage, patient characteristics, and specific medical conditions. Further research and clinical trials are needed to evaluate the efficacy and safety of suppository formulations for Chloroquine therapy.
ACKNOWLEDGEMENT
The authors are Thankful to for providing the facilities to carry out this research work in The Assam Kaziranga University, Jorhat, India.
- Newton CR, Sanjeev K (1998) Severe Falciparum Malaria in Children: Current Understanding of Pathophysiology and Supportive Treatment. Pharmacol Ther 79(1): 1-53.
- Summer AP, Stauffer WM, Fischer PR (2005) Pediatric malaria in the developing world. Semin Pediatr Infect Dis 16(2): 105-115.
- Samy EM, Hassan MA, Tous SS, Rhodes CT. Improvement of availability of allopurinol from pharmaceutical dosage forms I-suppositories. Eur J Pharm Biopharm 48(2): 119-127.
- Michael AO, Kolawole TJ (2004) Effects of interacting variables on the release properties of Chloroquine and aminophylline suppositories. Trop J Pharm Res 3(1): 285-290.
- Matsumoto K, Kimura S, Takahashi K, Yokoyama Y, Miyazawa M, et al. (2016) Pharmaceutical studies on and clinical application of olanzapine suppositories prepared as a hospital preparation. J Pharm Health Care Sci 2(1): 1-7.
- Omotunde O, Okubanjo1, Oluwatoyin AO (2009) Effect of interacting variables on the mechanical and release properties of Chloroquine phosphate suppositories. Acta Pharm Sciencia 51: 281-288.
- Antia-Obong OE, Ambrose AA, Alaribe M, Young U, Asuquo B, et al. (1995) Chloroquine phosphate suppositories in the treatment of childhood malaria in Calabar, Nigeria. Curr Ther Res 56(9): 927-935.
- Saleem MA, Taher M, Sanaullah S, Najmuddin M, Ali J, et al. (2008) Formulation and evaluation of tramadol hydrochloride rectal suppositories. Indian J Pharm Sci 70(5): 640.
- Tjoeng MM, Hogeman PH, Kapelle H, De Ridder ML, Verhaar H (1991) Comparative bioavailability of rectal and oral formulations of Chloroquine. Pharm Weekbl Sci 13(4): 176-178.
- Kawasaki C, Nishi R, Otagiri M (1997) Preparation and evaluation of a suppository dosage form containing Omeprazole. Pharm Sci 3: 431-434.
- Sah ML, Saini TR (2008) Formulation Development and Release Studies of Indomethacin Suppositories. Indian J Pharm Sci 70(4): 498-501.
- Mahjabeen S, Hatipoglu MK, Chandra V, Benbrook DM, Garcia-Contreras L (2018) Optimization of a vaginal suppository formulation to deliver SHetA2 as a novel treatment for cervical dysplasia. J Pharm Sci 107(2): 638-646.
- Oneyiji CO, Abedaya AS, Babalola CP (1999) Effects of absorption enhancers in Chloroquine suppository formulations. Eur J Pharm Sci 9(2): 131-136.
- D’souza AA, Shegokar R (2016) Polyethylene glycol (P.E.G.): A versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv 13(9): 1257-1275.
- Moghimipour E, Mohammad AD, Zarif F (2009) Characterization and in vitro evaluation of piroxicam suppositories. Asian J Pharm Clin Res 2(3): 92-98.
- Varshney HM, Tanwar YS (2009) Designing, Release Characteristics and in vitro Evaluation of Flurbiprofen Sodium Suppositories. Int J Pharm Clin Res 1(1): 31-34.
- Chinedum PB, Adebayob AS, Omotosoa A, Olubukola A (2004) Comparative bioavailability study of a new quinine suppository and oral quinine in healthy volunteers. Trop J Pharm Res 3(1):291-297.
- Taha EI, Zaghloul AA, Samyb AM, Al-Saidan S, Kassem AA, et al. (2004) Bioavailability assessment of salbutamol sulfate suppositories in human volunteers. Int J Pharm 279: 3-7.
- Regdon GJR, Schirm I, Pittmann A, Regdon G (1995) Production technology and in-vitro study of rectal suppositories containing Chloroquine Phosphate. Acta Pharm Hung 65(2): 45-50.
- Shanmugam S, Kim YH, Park JH, Im HT, Sohn YT, et al. (2014) Sildenafil vaginal suppositories: preparation, characterization, in vitro and in vivo Drug Dev Ind Pharm 40(6): 803-812.